Do you find yourself captivated by the invisible architecture of molecules, pondering how their shapes dictate their behavior? Understanding molecular geometry is key to unlocking the secrets of chemical reactions, material properties, and the very essence of matter.
We often find ourselves searching for one single place to gather all essential information, news, articles, comparisons, reviews, and top charts. That's precisely why this resource was created: to be a harbor for curious minds, a comprehensive hub where you can explore the fascinating world of molecular geometry. This article will delve into the intricacies of molecular shapes, exploring how atoms arrange themselves in three-dimensional space and how this arrangement impacts their interactions.
Another way of looking at molecular geometries is through the "AXE method" of electron counting. "A" in AXE represents the central atom and always has an implied subscript one; "X" represents the number of sigma bonds between the central and outside atoms (multiple covalent bondsdouble, triple, etc.count as one X); and "E" represents the number of lone electron pairs.
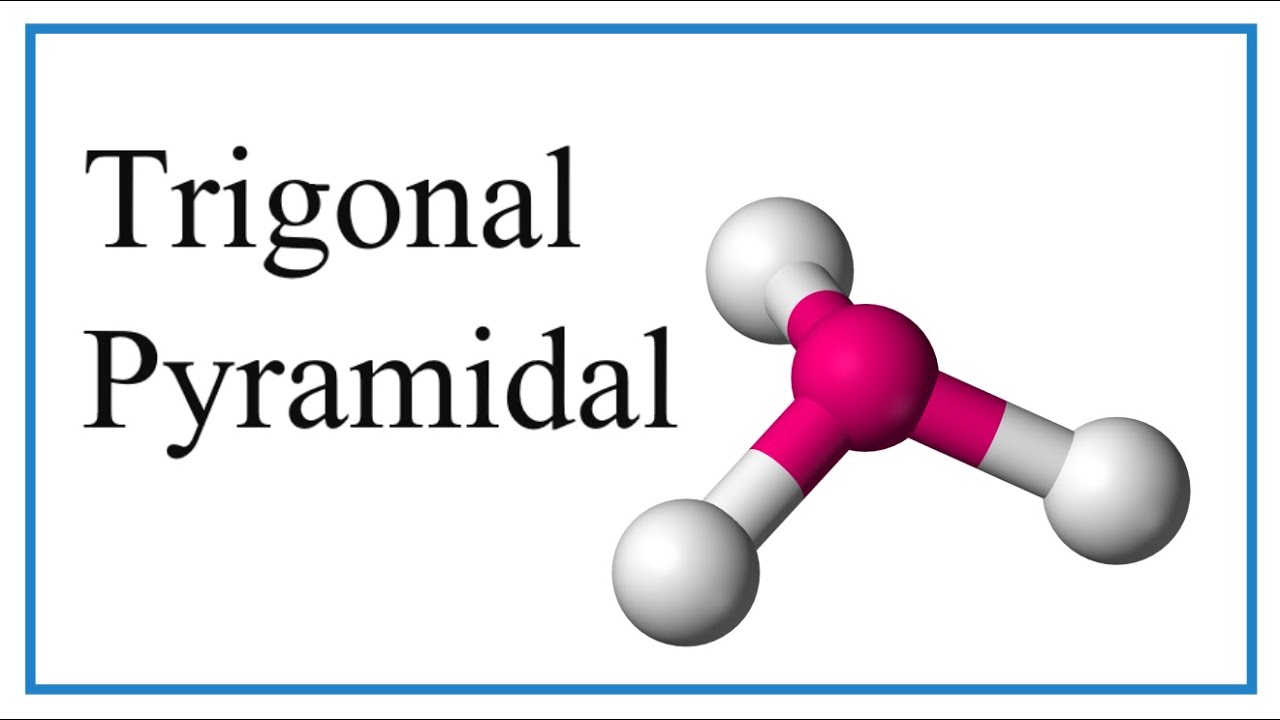
For example, consider the molecule with the formula AB3, its molecular shape is determined by the principles of Valence Shell Electron Pair Repulsion (VSEPR) theory. Let's explore a specific example: ammonia (NH3). Ammonia consists of three bond pairs and one lone pair. The lone pair exerts a repulsive force on the bond pairs, pushing them outwards and reducing the bond angle. This causes the bond angle to be slightly smaller than the idealized tetrahedral angle of 109.5, typically around 107. The resultant molecular shape of ammonia is trigonal pyramidal.
A trigonal pyramidal molecule has a steric number of 4, just like a tetrahedral molecule. The central atom has three bonds and one lone pair. In contrast, a tetrahedral molecule has four bonds and no lone pairs. A trigonal pyramidal has three bonds and one lone pair, so where a tetrahedral has regularly spaced bonds pointing towards the corners of a tetrahedron, the presence of a lone pair in the trigonal pyramidal molecule distorts the bond angles.
According to VSEPR theory, the molecular geometry of XeF2 is described as linear. The shape of an AX3E molecule is trigonal pyramidal. A molecule containing a central atom with sp3d2 hybridization has an octahedral electron geometry.
Let's consider the specific example of Phosphorus tribromide (PBr3). The molecular geometry of PBr3 is trigonal pyramidal. If the central atom has a double bond, a single bond, and one lone pair around it, the type of molecular geometry present is bent. When determining molecular shape, it's crucial to consider both the electron domain geometry and the arrangement of atoms.
If an atom has four electron domains, the electron domain geometry is tetrahedral, and the domains are approximately 109.5 degrees from each other. The shape of molecules like SOCl2 (sulfur is the central atom) is also determined by VSEPR theory. When considering the carbon atom of a carbonyl group, the VSEPR geometry is trigonal planar. To predict the molecular geometry of H3O+, we use VSEPR theory.
The bond dipole moments in some molecules, like those with trigonal pyramidal geometry, cannot cancel one another, and the molecule has a dipole moment. The bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than the single bonds, causing the HNH angle to be slightly smaller than 109.5.
Trigonal pyramidal geometry is a fundamental concept in chemistry that describes the shape of molecules with four electron pairs around a central atom, where one of the pairs is a lone pair. This molecular geometry plays a crucial role in determining the physical and chemical properties of various substances. An example of trigonal pyramid molecular geometry that results from tetrahedral electron pair geometry is NH3.
The nitrogen has 5 valence electrons and thus needs 3 more electrons from 3 hydrogen atoms to complete its octet. This leaves a lone electron pair that is not bonded to any other atom. This lone pair repels the bonding pairs, leading to a pyramidal shape.
By understanding these concepts, we gain the tools to predict and explain the shapes of molecules, which is vital for comprehending their behavior and function. From the basic principles of electron repulsion to the complexities of bond angles and hybridization, this exploration provides a foundation for deeper understanding in the fascinating world of molecular geometry.
| Category | Description |
|---|---|
| Molecular Geometry | The three-dimensional arrangement of atoms within a molecule. |
| VSEPR Theory | Valence Shell Electron Pair Repulsion theory, used to predict molecular shapes. |
| AXE Method | A method of electron counting where A = central atom, X = number of sigma bonds, and E = number of lone pairs. |
| Steric Number | The sum of the number of atoms bonded to the central atom and the number of lone pairs on the central atom. |
| Trigonal Pyramidal | A molecular shape with three atoms bonded to a central atom and one lone pair, such as NH3. |
| Tetrahedral | A molecular shape with four atoms bonded to a central atom, such as CH4. |
| Bond Angle | The angle formed between two bonds to a central atom. |
| Electron Domain Geometry | The arrangement of electron pairs around the central atom. |
| Hybridization | The mixing of atomic orbitals to form new hybrid orbitals. |
| Dipole Moment | A measure of the polarity of a molecule. |
| NH3 (Ammonia) | A molecule with trigonal pyramidal geometry. |
| PBr3 (Phosphorus Tribromide) | A molecule with trigonal pyramidal geometry. |
For further in-depth information and interactive models, consult reputable chemistry resources. Here's a link to a credible source: LibreTexts Chemistry - Molecular Shapes and Polarity. This will help with more accurate learning of the topic.